
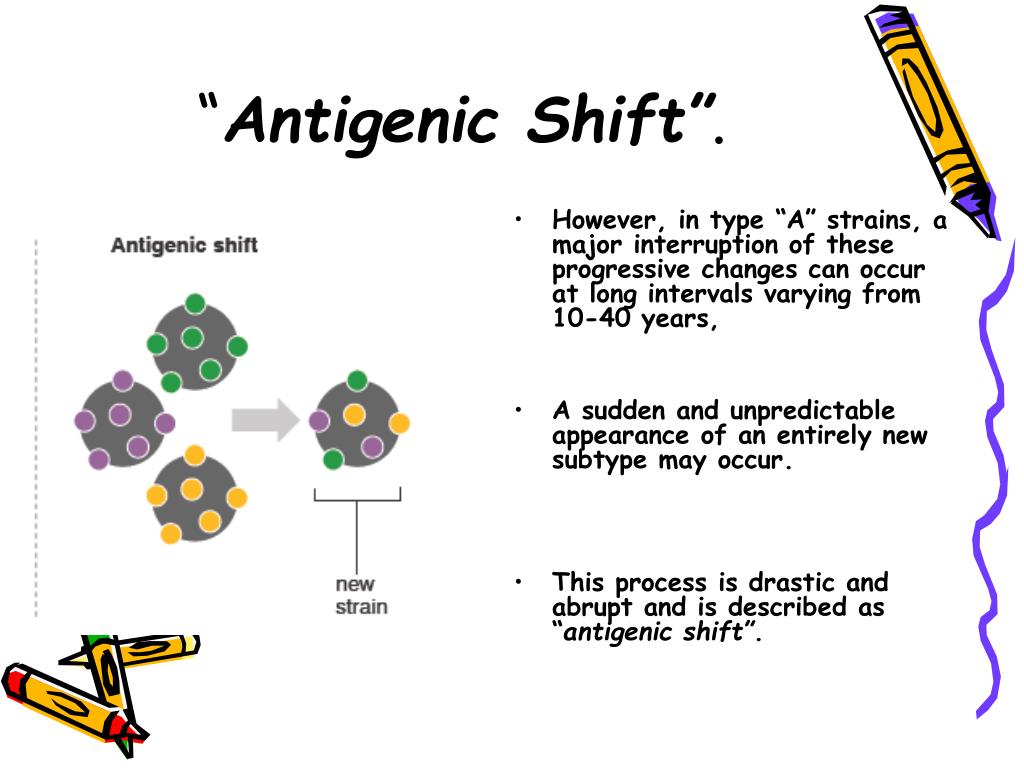
These variations are attributed to genome instability, and the lack of mechanisms for repairing RNA-dependent RNA polymerase errors occurs during replication in infected cells. The “antigenic drift” variations constantly cause minor and progressive changes in the structure of the HA and NA proteins of the influenza virus strains. They are known to have two distinct types of antigenic variation: antigenic drift and antigenic shift ( Roberts, 2008). Moulay Mustapha Ennaji, in Emerging and Reemerging Viral Pathogens, 2020 Antigenic Variations

The pandemic response may have contributed to improved preparedness for influenza pandemics in general and reduced barriers to large-scale vaccine manufacture and vaccination program implementation. Overall, the experience with the 2009 pandemic demonstrated that adjuvanted pandemic influenza vaccines are immunogenic, effective, and have an acceptably low rate of adverse events. Notably, narcolepsy has never been linked to seasonal influenza vaccines. For reasons currently unknown, this was not observed in other countries using that vaccine. Cases of narcolepsy appeared to be associated with the AS03-adjuvanted pandemic H1N1 vaccine in Finland, Sweden, and Iceland. However, others experienced an increase in narcolepsy, a rare condition characterized by impaired ability to regulate sleep-wake cycles leading to daytime sleepiness and involuntary sleep episodes. In general, the pandemic vaccines of 2009 had a safety profile comparable to seasonal vaccines and unusual safety issues were not observed in most countries. The adjuvanted vaccines during the 2009 pandemic attained very good effectiveness in many studies. Compared to seasonal epidemics, there were relatively more severe infections in children and young adults than in the elderly.

The pandemic itself turned out to be relatively mild, though in some cases (especially in clinical risk groups) resulted in severe viral pneumonitis, bacterial sequelae, and death. Here, a vaccine was developed, produced, and administered over only 8 months – though large-scale production was not attained as quickly as initially hoped. Another appealing aspect of adjuvants is dose sparing (i.e., extending the supply and availability of vaccine doses through less requirement of viral protein per dose).Īt the time of writing, the most recent pandemic vaccine preparation effort was in the 2009 A(H1N1) pandemic. The use of aluminum or lipid emulsion adjuvants has often been deemed necessary to achieve sufficient immunogenicity. A high-pathogenicity donor virus would have to be grown under high-containment conditions, so manufacturers have turned to other methods, including recombinant techniques and baculovirus vector-expressed vaccine antigens in cell cultures. Regulators such as the FDA and the European medicines agency (EMA) have developed procedures for fast-track licensure of pandemic vaccines.Īmong other hurdles, pandemic vaccine producers face increased safety concerns. Ways to enhance preparedness between pandemics include the preparation of collections of candidate vaccine viruses such as H5, H7, and H9 viruses. A key point to understand regarding vaccine production is that especially in the age of air travel, pandemics may spread very rapidly, and without proper forward planning and production capacity, vaccines may not be available until it is too late (though with several waves of pandemic influenza activity spread over some time, the vaccine may be available for the second wave if not the first one). Much of what is said above about seasonal influenza vaccines applies to pandemic influenza vaccines. The occurrence of pandemics is inherently unpredictable but in recent history, influenza pandemics have been observed every couple of decades: 1918, 1957, 1968, and 2009. Influenza viruses have a considerable pandemic potential because of their capacity for antigenic shift, adaptation to infect multiple species, and their mode of transmission. Topi Turunen, in Encyclopedia of Virology (Fourth Edition), 2021 Pandemic Influenza Vaccines


 0 kommentar(er)
0 kommentar(er)
